The world of organic chemistry is a fascinating realm, filled with complex molecules and intricate structures. Among these, esters hold a special place, playing crucial roles in everything from the flavors and fragrances we enjoy daily to the polymers that shape our modern world. Understanding how to name esters is not just an academic exercise; it’s a gateway to unlocking the secrets of these versatile compounds. This comprehensive guide will take you on a journey through the art and science of naming esters, providing you with the knowledge and tools you need to confidently navigate this important area of organic nomenclature.
What are Esters? A Brief Introduction
Before we dive into the intricacies of naming, let’s establish a solid foundation. Esters are organic compounds formed by the reaction of a carboxylic acid and an alcohol. This reaction, known as esterification, typically involves the removal of a water molecule. The general structure of an ester is RCOOR’, where R and R’ represent alkyl or aryl groups. The presence of the ester functional group (-COO-) is what defines these compounds and gives them their unique properties.
Esters are known for their pleasant odors, often associated with fruits and flowers. This is why they are widely used in the fragrance and flavor industries. Beyond their aromatic qualities, esters also find applications in solvents, plastics, and pharmaceuticals. Their versatility makes them essential building blocks in various chemical processes.
The Importance of Nomenclature in Chemistry
Nomenclature, the system of naming chemical compounds, is the universal language of chemistry. It allows chemists worldwide to communicate effectively, regardless of their native language. A well-defined nomenclature system ensures clarity, precision, and avoids ambiguity. Without a standardized naming system, the study and application of chemistry would be chaotic and inefficient.
Think of it like learning a new language. You start with the alphabet, then build words, and eventually, you can construct complex sentences. Naming organic compounds follows a similar pattern. By understanding the basic rules, you can decipher the names of even the most complex molecules.
Breaking Down the Naming Convention: The Basics
The naming of esters follows a systematic approach based on the IUPAC (International Union of Pure and Applied Chemistry) nomenclature rules. The name of an ester is derived from the names of the alcohol and the carboxylic acid used in its formation. The process involves two main steps:
- Identify the Alcohol Component: The alcohol component provides the first part of the ester’s name. This is the alkyl or aryl group attached to the oxygen atom in the ester functional group. The alcohol’s name is written first, followed by a space.
- Identify the Carboxylic Acid Component: The carboxylic acid component provides the second part of the ester’s name. This is the part of the molecule that contributes the carbonyl group (C=O) and the adjacent carbon atom. The name of the carboxylic acid is modified by dropping the “-oic acid” ending and replacing it with “-oate”.
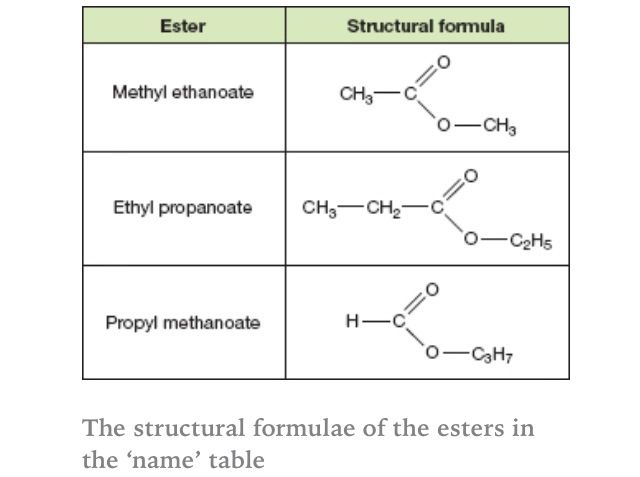
Let’s illustrate this with a simple example. Consider the ester formed from ethanol (an alcohol) and ethanoic acid (also known as acetic acid, a carboxylic acid). The resulting ester is ethyl ethanoate (or ethyl acetate). The “ethyl” comes from the ethanol, and the “ethanoate” comes from the ethanoic acid.
Step-by-Step Guide to Naming Esters
Now, let’s break down the process with more detailed steps and examples:
- Identify the Carboxylic Acid: Begin by recognizing the parent carboxylic acid. Determine the longest carbon chain containing the carbonyl group (C=O). This chain will form the base name.
- Identify the Alcohol: Determine the alkyl or aryl group attached to the oxygen atom in the ester functional group (-COO-). This group will form the first part of the ester’s name.
- Name the Ester: Combine the names. The alcohol group is written first, followed by the modified name of the carboxylic acid (dropping “-oic acid” and adding “-oate”). Use spaces to separate the names.
- Numbering (If Necessary): If the ester contains substituents (atoms or groups attached to the carbon chain), number the carbon atoms in the main chain, starting from the carbonyl carbon (C=O). Use numbers to indicate the positions of the substituents.
Example 1: Ethyl Acetate (Ethyl Ethanoate)
This is a classic example. As mentioned earlier, it’s formed from ethanol and ethanoic acid (acetic acid). Therefore, the name is ethyl ethanoate. The “ethyl” comes from the alcohol (ethanol), and the “ethanoate” comes from the carboxylic acid (ethanoic acid).
Example 2: Methyl Butanoate
Here, the alcohol component is methanol (methyl group), and the carboxylic acid component is butanoic acid. Following the rules, we get methyl butanoate. The “methyl” comes from the methanol, and the “butanoate” comes from the butanoic acid.
Example 3: Propyl Propanoate
In this case, the alcohol is propanol (propyl group), and the carboxylic acid is propanoic acid. The resulting ester is propyl propanoate.
Naming Esters with Complex Structures
As the structures of esters become more complex, the naming process becomes slightly more involved. Here are some additional considerations:
Branched Chains
If the carbon chain in either the alcohol or the carboxylic acid component is branched, you’ll need to identify the longest carbon chain and number the carbon atoms, just like you would for other organic compounds. Use prefixes like “methyl-”, “ethyl-”, “propyl-”, etc., to indicate the substituents and their positions.
Substituents on the Carboxylic Acid Chain
If there are substituents on the carbon chain of the carboxylic acid, you must indicate their positions using numbers. The carbonyl carbon (C=O) is always assigned the number 1. For example, 2-methylpropanoic acid would form an ester that needs the substituent location specified in the name of the ester.
Cyclic Esters (Lactones)
Cyclic esters, also known as lactones, are named in a similar way, but the name reflects the cyclic structure. Lactones are formed when a carboxylic acid and an alcohol group are within the same molecule. The name is derived from the corresponding carboxylic acid, but the “-oic acid” ending is replaced with “-olactone”. For example, a five-membered ring lactone derived from a carboxylic acid containing five carbons would be named a “pentanolactone.” The position of the carbonyl group is usually assumed to be at position 1.
Esters with Multiple Functional Groups
When an ester contains other functional groups (e.g., alcohols, ketones, or amines), the naming becomes more complex. The ester group is generally considered the principal functional group, and the other groups are treated as substituents. Prioritize the functional groups based on their priority in the IUPAC nomenclature. This can get complicated, and a good understanding of functional group priorities is essential.
Common Mistakes to Avoid When Naming Esters
Even experienced chemists sometimes make mistakes. Here are some common pitfalls to watch out for:
- Mixing up the Alcohol and Carboxylic Acid Components: Always remember that the alcohol component comes first in the name.
- Incorrectly Identifying the Parent Chain: Ensure you identify the longest carbon chain containing the carbonyl group of the carboxylic acid.
- Forgetting to Number Substituents: If there are substituents, don’t forget to number the carbon atoms in the main chain and indicate the positions of the substituents.
- Incorrectly Modifying the Carboxylic Acid Name: Remember to replace the “-oic acid” ending with “-oate”.
- Ignoring IUPAC Rules: Always adhere to the IUPAC nomenclature guidelines to ensure accuracy and consistency.
Practical Tips for Mastering Ester Nomenclature
Learning to name esters effectively requires practice and a systematic approach. Here are some tips to help you succeed:
- Practice, Practice, Practice: The more examples you work through, the more comfortable you’ll become with the naming conventions.
- Use Flashcards: Create flashcards with the structure of an ester on one side and its name on the other.
- Work Through Examples: Find examples in textbooks, online resources, or create your own.
- Use Online Nomenclature Tools: Many online tools can help you check your work and provide feedback.
- Seek Help When Needed: Don’t hesitate to ask your instructor, classmates, or online forums for help if you’re struggling.
- Understand the Underlying Concepts: Focus on understanding the fundamental principles of organic chemistry, not just memorizing rules.
- Relate to Real-World Examples: Connect the names of esters to their applications in flavors, fragrances, and other products.
Advanced Topics and Further Exploration
Once you have mastered the basics, you can delve into more advanced topics:
- Nomenclature of Complex Esters: Explore the naming of esters with multiple ester groups, cyclic esters, and those with other functional groups.
- Spectroscopic Techniques: Learn how techniques like NMR and IR spectroscopy can be used to confirm the structure of esters.
- Reactions of Esters: Study the chemical reactions that esters undergo, such as hydrolysis, saponification, and transesterification.
- Industrial Applications: Research the various industrial applications of esters, including their use in plastics, solvents, and pharmaceuticals.
Conclusion: Embracing the Beauty of Ester Nomenclature
Naming esters might seem daunting at first, but with a systematic approach and consistent practice, you can master this essential skill. By understanding the rules and applying them diligently, you’ll be able to confidently name and understand the structures of a wide range of esters. This knowledge will not only enhance your understanding of organic chemistry but also open doors to a deeper appreciation of the fascinating world of molecules and their applications.
Remember, the key is to break down the process into manageable steps, practice regularly, and seek help when needed. As you gain experience, you’ll find that naming esters becomes second nature. So, embrace the challenge, explore the beauty of ester nomenclature, and unlock the secrets of these remarkable compounds!
By mastering the art of naming esters, you’re not just learning a set of rules; you are acquiring a valuable skill that will serve you well in your journey through organic chemistry and beyond. Happy naming!

