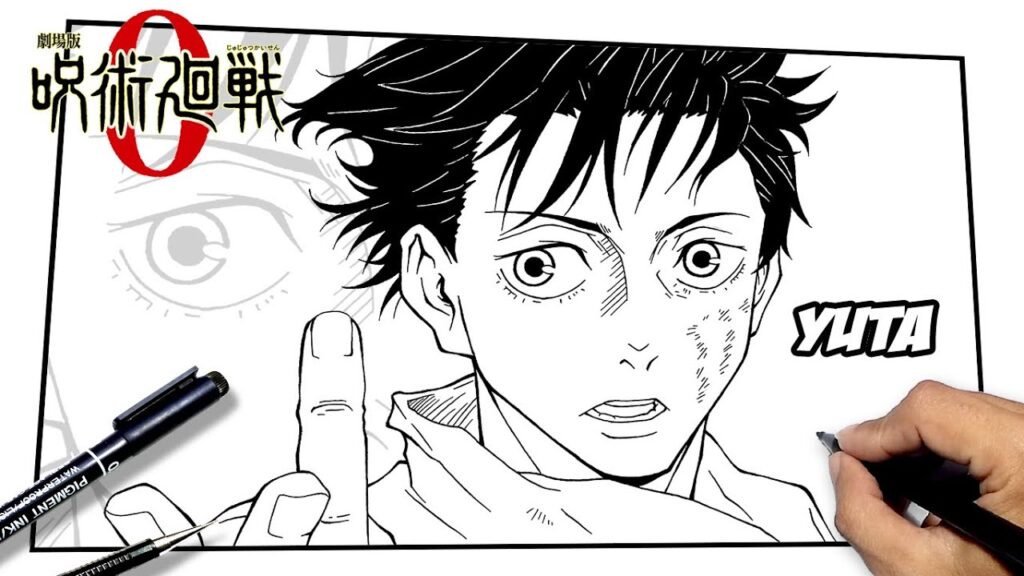
Introduction: Embark on Your Artistic Journey with Yuta Okkotsu
So, you’re a fan of Jujutsu Kaisen and want to learn how to draw Yuta Okkotsu? Awesome! Yuta, with his complex backstory and compelling character design, is a fantastic subject for any aspiring artist. Whether you’re a seasoned pro or just starting out, this guide will break down the process into easy-to-follow steps, helping you capture Yuta’s essence on paper (or screen!). We’ll go beyond just drawing lines; we’ll delve into understanding his character, which will ultimately inform your artistic decisions and bring your drawing to life.
Why Yuta Okkotsu is a Great Subject
Before we dive into the technical aspects, let’s talk about why Yuta is such a compelling character to draw. His design is a blend of vulnerability and strength. His eyes often reflect a deep sadness, hinting at the burdens he carries, while his posture and expressions can shift to portray unwavering resolve. He’s not just another anime pretty boy; he’s a character with depth, and that depth translates beautifully into art.
Furthermore, Yuta’s design offers a good balance of complexity and simplicity. His hair, while distinct, isn’t overly intricate. His clothing is relatively straightforward, allowing you to focus on capturing his facial features and overall demeanor. He provides a challenge without being overwhelming, making him an excellent subject for artists of all skill levels.
Materials You’ll Need
Don’t worry, you don’t need a fancy art studio to get started. Here’s a list of basic materials that will work just fine:
- Pencils: A range of pencils, from 2H (for light sketching) to 2B or 4B (for darker lines and shading), is ideal.
- Eraser: A kneaded eraser is great for lifting graphite without damaging the paper, while a regular eraser is good for more thorough corrections.
- Paper: Smooth drawing paper is recommended. Avoid paper that is too textured, as it can make it difficult to achieve clean lines.
- Optional:
- Ruler: Helpful for creating guidelines and ensuring proportions are accurate.
- Blending Stump or Tortillon: Useful for creating smooth transitions in your shading.
- Reference Images: Crucial for capturing Yuta’s likeness. Gather various images of him from different angles and expressions.
Step-by-Step Guide to Drawing Yuta Okkotsu
1. Laying the Foundation: Basic Shapes and Proportions
Every great drawing starts with a solid foundation. Begin by sketching light, basic shapes to map out Yuta’s head and body. Think circles, ovals, and rectangles. These shapes will act as placeholders, helping you establish the overall proportions and pose. Don’t worry about perfection at this stage; focus on getting the general structure right.
- Head: Start with a circle for the cranium. Add a slightly flattened oval below for the jawline.
- Body: Use a simplified rectangle for the torso and elongated ovals for the limbs.
- Guidelines: Draw a vertical line down the center of the face to ensure symmetry. Add horizontal lines to indicate the placement of the eyes, nose, and mouth.
Remember, these are just guidelines. Don’t press too hard with your pencil, as you’ll be erasing them later.
2. Refining the Face: Capturing Yuta’s Likeness
Now comes the crucial part: defining Yuta’s facial features. Pay close attention to your reference images and focus on capturing the unique details that make him recognizable.
- Eyes: Yuta’s eyes are one of his most defining features. They’re slightly downturned, giving him a melancholic expression. Sketch the basic shape of the eyes, paying attention to the size and spacing. Add the pupils and irises, and don’t forget the highlights!
- Eyebrows: His eyebrows are relatively straight and thick, adding to his serious demeanor.
- Nose: The nose can be simplified to a few lines. Focus on capturing its shape and position relative to the other facial features.
- Mouth: Yuta’s mouth is often depicted with a subtle curve, suggesting a hint of sadness or determination.
- Hair: Yuta’s hair is a distinctive feature. It’s messy and slightly unkempt, with several strands falling across his forehead. Start by sketching the overall shape of the hair, then add in the individual strands.
Take your time with this step. The face is the focal point of the drawing, so it’s important to get it right.
3. Detailing the Body: Clothing and Posture
Once you’re satisfied with the face, move on to detailing the body. Pay attention to the folds and wrinkles in his clothing, and try to capture the overall posture. Is he standing tall and confident, or is he hunched over and withdrawn?
- Clothing: Yuta’s attire is typically his Jujutsu High uniform. Pay attention to the details of the uniform, such as the collar, buttons, and folds in the fabric.
- Hands: Hands can be tricky to draw, but they’re an important part of the overall composition. Practice drawing hands in different poses to improve your skills.
- Posture: The way Yuta stands or sits can convey a lot about his personality. Observe his posture in your reference images and try to replicate it in your drawing.
4. Shading and Highlights: Adding Depth and Dimension
Shading is what brings a drawing to life. It adds depth and dimension, making the character look more realistic. Observe the light and shadows in your reference images and try to replicate them in your drawing.
- Light Source: Determine the direction of the light source. This will help you decide where to place the shadows and highlights.
- Shading Techniques: Experiment with different shading techniques, such as hatching, cross-hatching, and blending.
- Highlights: Highlights are the areas where the light is hitting the surface directly. They add a sense of brightness and shine to the drawing.
Don’t be afraid to experiment with different shading techniques. The more you practice, the better you’ll become at creating realistic shading.
5. Line Weight and Final Touches: Polishing Your Masterpiece
Once you’re satisfied with the shading, it’s time to add the final touches. Go over your lines with a slightly darker pencil to define the contours and add emphasis to certain areas. This is also a good time to add any small details that you may have missed.
- Line Weight: Varying the line weight can add depth and interest to the drawing. Use thicker lines for the outlines and thinner lines for the details.
- Details: Add any small details that you may have missed, such as wrinkles in the clothing or stray hairs.
- Clean Up: Erase any remaining guidelines and stray marks.
Step back and take a look at your drawing. Are there any areas that need further refinement? Make any final adjustments and then sign your masterpiece!
Tips and Tricks for Drawing Anime Characters
- Practice Regularly: The more you practice, the better you’ll become. Dedicate some time each day to drawing, even if it’s just for a few minutes.
- Use Reference Images: Reference images are essential for capturing the likeness of a character. Gather as many images as you can from different angles and expressions.
- Study Anatomy: Understanding anatomy will help you draw more realistic figures.
- Experiment with Different Styles: Don’t be afraid to experiment with different styles and techniques.
- Don’t Give Up: Drawing can be challenging, but it’s also incredibly rewarding. Don’t get discouraged if your drawings don’t turn out perfect at first. Just keep practicing and you’ll eventually see improvement.
Common Mistakes to Avoid
- Ignoring Proportions: Accurate proportions are crucial for creating a believable drawing. Pay attention to the relative size and spacing of the different body parts.
- Using Too Much Pressure: Pressing too hard with your pencil can make it difficult to erase mistakes and can also create unwanted texture on the paper.
- Not Using Reference Images: Relying on your memory alone can lead to inaccuracies. Always use reference images to ensure that you’re capturing the character’s likeness.
- Giving Up Too Easily: Drawing can be frustrating at times, but it’s important to persevere. Don’t give up if your drawings don’t turn out perfect at first. Just keep practicing and you’ll eventually see improvement.
Advanced Techniques for Drawing Yuta
Dynamic Poses
Once you’re comfortable drawing Yuta in static poses, try experimenting with dynamic poses. This will add a sense of energy and movement to your drawings. Consider Yuta in action, wielding his sword or using his cursed technique. Observe how his body twists and bends, and try to capture that movement in your drawing.
Facial Expressions
Yuta is a character with a wide range of emotions. Practice drawing him with different facial expressions to convey his personality. Experiment with different eye shapes, eyebrow positions, and mouth shapes to create different emotions. Refer to various scenes from the anime or manga to capture the nuances of his expressions.
Adding Backgrounds
Adding a background can help to set the scene and add context to your drawing. Consider drawing Yuta in a battlefield, a classroom, or any other location that is relevant to the story. The background should complement the character and add to the overall composition of the drawing.
Digital Art
If you’re interested in taking your art to the next level, consider trying digital art. Digital art allows you to create drawings and paintings using a computer or tablet. There are many different software programs available, such as Adobe Photoshop, Clip Studio Paint, and Procreate. Digital art offers a wide range of tools and features that can help you create stunning artwork.
Where to Find Inspiration and Resources
- Jujutsu Kaisen Anime and Manga: The anime and manga are the best sources of inspiration for drawing Yuta. Pay attention to the character designs, poses, and expressions.
- Online Art Communities: There are many online art communities where you can share your artwork, get feedback, and learn from other artists. Websites like DeviantArt and ArtStation are great places to start.
- Art Tutorials: There are countless art tutorials available online, both free and paid. These tutorials can teach you everything from basic drawing techniques to advanced shading methods.
- Art Books: Art books can be a great source of inspiration and information. Look for books on anatomy, perspective, and character design.
Beyond the Basics: Exploring Yuta’s Character Through Art
Drawing Yuta isn’t just about replicating his appearance; it’s about understanding his character and conveying his emotions through your art. Consider his backstory, his relationships with other characters, and his motivations. How does his past trauma affect his expressions and posture? How does his bond with Rika manifest in his actions? By exploring these aspects of his character, you can create drawings that are not only visually appealing but also emotionally resonant.
Think about the weight of Rika’s curse on him, the loneliness he initially felt, and the gradual growth he experiences as he connects with his classmates. These emotions can be subtly conveyed through your art, adding layers of depth to your depiction of Yuta.
Conclusion: Keep Practicing and Have Fun!
Learning to draw Yuta Okkotsu takes time and practice, but it’s a rewarding experience. By following the steps outlined in this guide and continuing to practice, you’ll be able to create stunning drawings of your favorite Jujutsu Kaisen character. Remember to have fun and experiment with different styles and techniques. The most important thing is to enjoy the process and let your creativity flow!
So grab your pencils, gather your reference images, and start drawing! The world of Jujutsu Kaisen awaits your artistic interpretation. Good luck, and happy drawing!

