How to Find Oxidation Numbers: A Comprehensive Guide
Determining oxidation numbers (also known as oxidation states) might seem daunting at first, but with a systematic approach, it becomes straightforward. This guide will equip you with the knowledge and techniques to confidently calculate oxidation numbers for various elements and compounds. Understanding oxidation numbers is crucial in chemistry, particularly in redox reactions and balancing chemical equations.
Understanding Oxidation Numbers
Before diving into the calculation methods, let's clarify what oxidation numbers represent. The oxidation number is a hypothetical charge assigned to an atom in a molecule or ion, assuming that all bonds are completely ionic. It indicates the degree of oxidation (or reduction) of an atom in a compound. It's important to remember that oxidation numbers are not real charges; they're a bookkeeping tool to track electron transfer.
Rules for Assigning Oxidation Numbers
Several rules govern the assignment of oxidation numbers. These rules are applied sequentially, with later rules overriding earlier ones if conflicts arise.
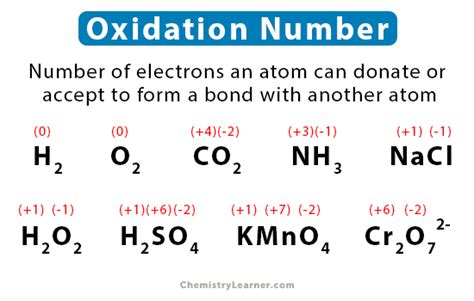
Rule 1: The oxidation number of an element in its free (uncombined) state is always 0.
- Example: The oxidation number of O₂ (oxygen gas) is 0, and the oxidation number of Na (sodium metal) is 0.
Rule 2: The oxidation number of a monatomic ion is equal to its charge.
- Example: The oxidation number of Na⁺ (sodium ion) is +1, and the oxidation number of Cl⁻ (chloride ion) is -1.
Rule 3: The oxidation number of hydrogen is +1, except in metal hydrides where it is -1.
- Example: In H₂O (water), hydrogen has an oxidation number of +1. In NaH (sodium hydride), hydrogen has an oxidation number of -1.
Rule 4: The oxidation number of oxygen is usually -2, except in peroxides (where it is -1) and in compounds with fluorine (where it is positive).
- Example: In H₂O (water), oxygen has an oxidation number of -2. In H₂O₂ (hydrogen peroxide), oxygen has an oxidation number of -1.
Rule 5: The oxidation number of a group 1 (alkali metals) element is always +1.
- Example: The oxidation number of Li in LiCl is +1.
Rule 6: The oxidation number of a group 2 (alkaline earth metals) element is always +2.
- Example: The oxidation number of Mg in MgCl₂ is +2.
Rule 7: The sum of the oxidation numbers of all atoms in a neutral molecule is 0.
- Example: In H₂O, the sum of the oxidation numbers is (+1)x2 + (-2) = 0.
Rule 8: The sum of the oxidation numbers of all atoms in a polyatomic ion is equal to the charge of the ion.
- Example: In SO₄²⁻ (sulfate ion), the sum of the oxidation numbers must equal -2.
Calculating Oxidation Numbers: Worked Examples
Let's apply these rules to some examples:
Example 1: Finding the oxidation number of sulfur in H₂SO₄ (sulfuric acid).
- We know the oxidation number of hydrogen is +1 (Rule 3) and oxygen is -2 (Rule 4).
- Let 'x' represent the oxidation number of sulfur.
- Using Rule 7 (sum of oxidation numbers in a neutral molecule is 0): 2(+1) + x + 4(-2) = 0
- Solving for x: x = +6. Therefore, the oxidation number of sulfur in H₂SO₄ is +6.
Example 2: Finding the oxidation number of manganese in MnO₄⁻ (permanganate ion).
- We know the oxidation number of oxygen is -2 (Rule 4).
- Let 'x' represent the oxidation number of manganese.
- Using Rule 8 (sum of oxidation numbers in a polyatomic ion equals the charge): x + 4(-2) = -1
- Solving for x: x = +7. Therefore, the oxidation number of manganese in MnO₄⁻ is +7.
Tips for Success
- Practice: The key to mastering oxidation number calculations is practice. Work through numerous examples to build your understanding.
- Systematic Approach: Follow the rules sequentially and methodically.
- Check Your Work: Always verify that the sum of the oxidation numbers equals zero for neutral molecules or the ion's charge for polyatomic ions.
By following these rules and practicing regularly, you'll become proficient in determining oxidation numbers, a fundamental skill in chemistry. Remember to always consult a periodic table for the elements' symbols and group numbers.