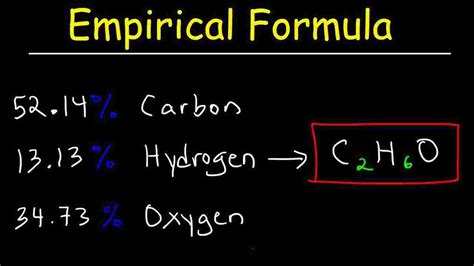
How to Get the Empirical Formula: A Step-by-Step Guide
Determining the empirical formula of a compound is a fundamental concept in chemistry. The empirical formula represents the simplest whole-number ratio of atoms in a compound. This guide will walk you through the process, explaining each step clearly and concisely. We'll cover everything from understanding the basics to tackling more complex scenarios.
Understanding the Basics: What is an Empirical Formula?
Before diving into the calculations, let's solidify our understanding. The empirical formula shows the ratio of elements present in a compound in their simplest form. It doesn't necessarily represent the actual number of atoms in a molecule (that's the molecular formula), but rather the smallest whole-number ratio.
For example, the molecular formula of glucose is C₆H₁₂O₆. Its empirical formula, however, is CH₂O, because the ratio of carbon, hydrogen, and oxygen atoms is 1:2:1.
Step-by-Step Guide to Determining the Empirical Formula
To determine the empirical formula, you generally need the mass percentages of each element present in the compound. Here's a step-by-step process:
Step 1: Assume a 100g Sample
This simplifies the calculations. If you are given percentages, assuming a 100g sample means the percentage of each element directly translates to its mass in grams. For example, if a compound is 40% carbon, in a 100g sample, there would be 40g of carbon.
Step 2: Convert Grams to Moles
Use the molar mass of each element (found on the periodic table) to convert the mass of each element (in grams) to moles. Remember the formula:
Moles = Mass (g) / Molar Mass (g/mol)
Step 3: Find the Mole Ratio
Divide the number of moles of each element by the smallest number of moles calculated in Step 2. This will give you the simplest whole-number ratio of the elements.
Step 4: Express as a Whole Number Ratio
If the mole ratios are not whole numbers (e.g., 1.5, 2.5), you need to multiply all the ratios by a small integer to obtain whole numbers. For example, if you have a ratio of 1.5:1, multiply both by 2 to get 3:2.
Step 5: Write the Empirical Formula
Use the whole-number ratios obtained in Step 4 to write the empirical formula. The subscripts represent the number of atoms of each element.
Example: Finding the Empirical Formula of a Compound
Let's say a compound contains 75% carbon and 25% hydrogen. Let's find its empirical formula:
-
Assume a 100g sample: 75g Carbon, 25g Hydrogen.
-
Convert to moles:
- Moles of Carbon: 75g / 12.01 g/mol (molar mass of Carbon) ≈ 6.24 mol
- Moles of Hydrogen: 25g / 1.01 g/mol (molar mass of Hydrogen) ≈ 24.75 mol
-
Find the mole ratio: Divide by the smallest number of moles (6.24 mol):
- Carbon: 6.24 mol / 6.24 mol = 1
- Hydrogen: 24.75 mol / 6.24 mol ≈ 3.96 ≈ 4 (rounding to the nearest whole number is acceptable for empirical formulas)
-
Whole-number ratio: The ratio is already 1:4.
-
Empirical formula: CH₄ (Methane)
Beyond the Basics: Handling More Complex Scenarios
Sometimes, you might be given information other than mass percentages, such as the mass of the compound and the mass of each element within it. The process remains largely the same; you still need to convert everything to moles and find the simplest whole-number ratio.
Remember to always double-check your calculations and consider the context of the problem. Accurate molar masses are crucial for accurate results.
Conclusion: Mastering Empirical Formula Calculations
Determining the empirical formula is a crucial skill in chemistry. By following these steps and understanding the underlying principles, you can confidently tackle this type of problem and improve your understanding of chemical composition. Remember to practice regularly with different examples to solidify your understanding.